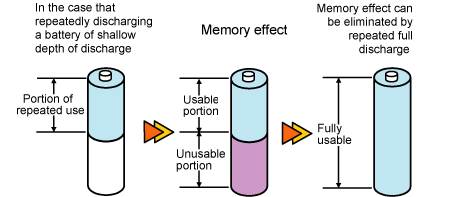
Tulevaisuuden akut tarjoilevat lupaavat ennennäkemätöntä suorituskykyä. Oletko koskaan miettinyt, miksi ladattava paristosi on juuri litiumionia tai litiumpolymeeriä? Miten akkuja voi kehittää?
Erityisesti litiumioniakut ovat kantaneet akkuteollisuutta niiden tultua markkinoille 1990-luvulla. Tämän jälkeen akkujen kehitys on pohjautunut enimmäkseen litiumioni akkujen parantamiseen, mutta varsinainen kehitysloikka on antanut odottaa itseään miltei 30 vuotta. Kyse ei ole yrittämisen puutteesta, vaan markkinoiden luomista tiukoista vaatimuksista. Ilmoituksia uusista innovaatioista tulee viikoittain, mutta useimmat ovat taloudellisesti kannattamattomia tai liian epävakaita laajaan käyttöön.
Missä viipyy sähköauto 1000 km toimintamatkalla? – Akun valmistamiseen liittyvät haasteet
Teknologinen innovointi ei yksinään riitä, jos akusta ei saada tehtyä tarpeeksi edullista valmistaa. Menestyäkseen markkinoilla akkujen täytyy läpäistä tiukat turvallisuuskriteerit sekä tarjota riittävä kapasiteetti/kestävyys suhde hintaan nähden.
Akkujen tuotteistaminen
Akun toimivuutta ja käytettävyyttä arvioitaessa keskitytään kahdeksaan osa-alueeseen, joissa kaikissa akun tulee täyttää vaaditut kriteerit. Nämä osa-alueet ovat:
1) Kapasiteetti.
Mitä pidemmän aikaa akussa riittää virtaa latauksen jälkeen, sitä parempi. Tätä osa-aluetta kuvaa akun ampeeritunti Ah. Mitä isompi Ah on, sitä kauemmin akku tuottaa virtaa. Kapasiteettia lisätään yleensä purkuvirran kustannuksella ja toisinpäin.
2) Purkuvirta.
Tämä kertoo, kuinka paljon virtaa akku on suunniteltu luovuttamaan käytössä. Sähköiset työkalut vaativat yleensä suuremman purkuvirran, jonka vuoksi akkujen kapasiteetti puolestaan on pienempi.
3) Edullinen hinta.
Valmistamisen muodostamat kulut pyritään peittoamaan massatuotannolla. Massatuotanto puolestaan vaatii, että akulle on tiedossa laaja käyttäjäkunta markkinoilla. Uusi teknologia nostaa aluksi tuotantokustannuksia, erityisesti korkean suorituskyvyn akuissa.
4) Pitkä käyttöikä.
Käyttöikä on yksi tärkeimpiä osa-alueita koskien erityisesti isoja, kalliimpia akkupaketteja. Nykyiset sähköautot saavat moitteita niiden akuista, jotka kestävät tällä hetkellä noin 8–10 vuotta. Mikäli näiden akkujen kestävyys saataisiin nostettua 20 vuoteen, sähköautojen käyttö olisi huomattavasti nykyistä kannattavampaa, vaikkakin kyseinen akku olisi hiukan kalliimpikin.
Nykyiset li-ion akut kestävät noin 500-1000 uudelleenlatausta riippuen käytöstä. Vanhempi akkukemia Ni-MH taas voi kestää 2000 uudelleenlataustakin, mutta ne painavat enemmän pienemmällä energiatiheydellä.
5) Turvallisuus.
Akkuihin liittyviä turvallisuusriskejä huomioidaan jatkuvasti aiempaa tarkemmin, jonka seurauksena myös käyttöturvallisuuden takaamiseksi on laadittu erilaisia säädöksiä akun toimivuutta koskien. Turvallisuusnäkökulma on yksi yleisin heikko lenkki uusissa kokeellisissa akuissa.
Esimerkiksi Li-Po akku voidaan muotoilla miltei mihin muotoon tahansa. Akussa on myös korkea energiatiheys (suuri kapasiteetti vs koko), mutta sen käyttöä rajoittaa mekaaninen heikkous. (katso alempi gif)

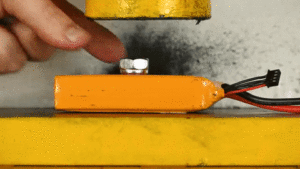
6) Laaja käyttöympäristö.
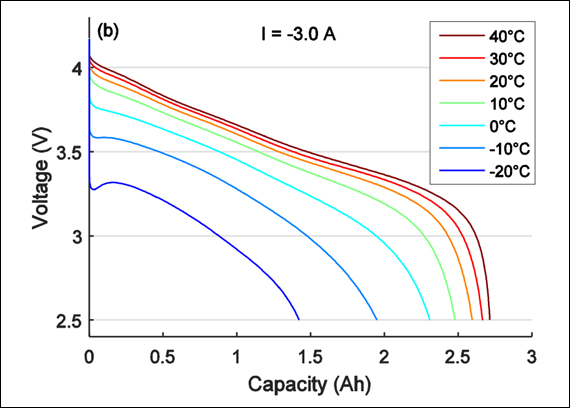
Yksikään akku ei kestä pitkiä aikoja kylmiä tai kuumia lämpötiloja. Valmistajat ovat suunnitelleet akuille suojaavia eristekerroksia paremman pakkaskestävyyden vuoksi, mutta nykyiset akkukemiat menettävät jokatapauksessa ominaisuuksia kuumassa ja kylmässä.
7) Myrkyllisyys.
Ympäristösyistä kadmium- ja elohopeapohjaiset akut ovat poistuneet jo käytöstä ja korvattu uusilla metalleilla. Euroopassa samaa yritetään myös lyijyhappoakuille, mutta tälle ei ole löydetty taloudellista korvaajaa, jolla olisi sama suorituskyky.
Nikkeli- ja litiumpohjaiset akut sisältävät vähän myrkyllistä materiaalia, mutta muodostavat silti saastumisvaaran, mikäli ne hävitetään huolimattomasti. Tulevaisuuden akun tulisikin sisältää entistä vähemmän ympäristölle haitallisia materiaaleja, joka muodostaa valmistajille suuren haasteen. Katso Proakku artikkeli akkujen kierrättämisestä tästä.

8) Nopea latausaika.
”Fast charging” eli pikalataus on akkujen uusin trendi. Kaikki akkumateriaalit eivät tue pikalatausta, kuten lyijyhappoakku. Pikalataus vaatii tarkat olosuhteet toimiakseen, esimerkiksi akun on oltava huoneenlämpöinen ja hyväkuntoinen. Korkeilla virroilla lataaminen kuluttaa akkua huomattavasti, ellei latausta tehdä ”älykkäästi,” esimerkiksi 80% varauksessa latausvirtaa tiputetaan akun säästämiseksi.
Valmistajien haasteena onkin sellaisen akun suunnittelu, joka mahdollistaa myös entistä nopeat latausajat tinkimättä akun käytettävyydestä tai turvallisuudesta.
Kaikki kriteerit täyttävä akku
Useat kehitteillä olevat akkuinnovaatiot täyttävät tai ylittävät tiettyjen osa-alueiden kriteerit, mutta jäävät vajaaksi toisilla. Tämä käytännössä estää akun myymisen kuluttajille ennen kuin akku kehitetään tasapainoisemmaksi.
Esimerkiksi litium-ilma (Li-air) teknologialla toimivan akun teoreettinen kapasiteetti olisi 13kWh/kg, kun litiumioniakun vastaava kapasiteetti olisi 265 Wh/kg – Li-air-akulla olisi siis melkein viisinkertainen kapasiteetti. Toisaalta akku kestäisi tällä hetkellä vain 50 lataussykliä, jonka vuoksi sitä ei kannata tuoda markkinoille.
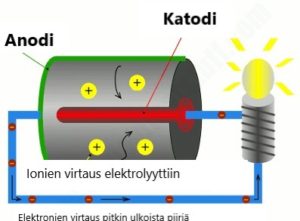
Mikäli haluat kerrata akkukemian merkityksen ja ymmärtää anodit ja katodit, lue artikkelimme: Kuinka pilaat akkusi
Myrkyttömyys vai akku, joka sitoo hiilidioksidia latautuessaan? – Tulevaisuuden akut

Akkuteollisuuden keskuudessa käydään tällä hetkellä kovaa kilpailua siitä, kuka saa markkinoille litiumioniakun korvaajan. Kilpailu on raakaa, sillä akkujen valmistamisessa tulee huomioida yhä enemmän ympäristöystävällisyys. Tämä vielä edellämainittujen kahdeksan kohdan lisäksi. Silti akkuprototyyppejä on jo valmistettu, joista osa on lupaavia.
Tässä muutama esimerkki akuista, joita saatamme nähdä lähivuosina:
Myrkytön sinkki-mangaani Zn–Mn
Australiassa patentoitu akku hyödyntää myrkyttömiä sinkkiä ja mangaania tuottaakseen korkean energiatiheyden omaavan akun. Akussa on myös vesipitoinen elektrolyytti, joka estää sen syttymisen palamaan. Akun hinnan arvioidaan olevan alle 9 euroa /kWh, kun nykyinen li-ion akku kustantaa noin 265 euroa / kWh.
Akku on tarkoitettu kohteisiin, joissa akun paino, koko ja turvallisuus ovat tärkeimmät tekijät – eli erityisesti autoihin, ilmailualuksiin kuin myös koti- ja liiketalouksiin. Jo olemassa olevista sinkki-mangaaniakuista tämä akku eroaa mahdollistamalla uudelleenlatauksen, kierrätyksen sekä korkean energiatiheyden.
Akun kehittäjän mukaan tarve varastoida kestävällä tavalla tuotettua energiaa turvallisesti ja kustannustehokkaasti kasvaa joka päivä. Nykyiset akkumateriaalit, kuten litium, lyijy ja kadmium, eivät pärjää varsinkaan ympäristöystävällisyydessä. Sinkki-mangaaniakku tuottaisi tavan varastoida energiaa suuremmalla skaalalla kuin nykyiset ratkaisut.
http://theleadsouthaustralia.com.au/industries/technology/new-battery-technology-could-slash-the-cost-of-electric-vehicles/
Hiilidioksidia sitova akku
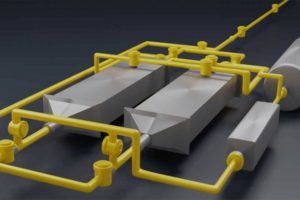
Mitä jos akkusi näyttäisikin tältä? Hiilidioksidia sitova akku.
Uusinta vihreää teknologiaa edustava, Massachusettsin Teknologian Instituutin (MIT) kehittämä, akkuratkaisu kykenee sitomaan itseensä hiilidioksidia ilmasta. Varsinaisen materiaalit eivät edusta tässä akussa uutta ja innovatiivista teknologiaa, vaan akun toimintaperiaate.
Akkukennot ovat rakennettu liuskamaisiksi, hieman erilleen toisistaan, mahdollistaakseen ilmavirran akuston läpi. Akun latautuessa sen läpi virtaa ilmaa, josta akkukennot pienen sähkövirran ja kemiallisen reaktion avustamana sitovat itseensä ilmavirran mukana olevaa hiilidioksidia. Näin ollen akun toisesta päästä tuleva ilma on puhtaampaa. Kun akku purkautuu, se luovuttaa siihen sitoutuneen hiilidioksidin pois. Tämä puhdas hiilidioksidi voidaan käyttää esimerkiksi kasvihuoneiden kasveille tai kuplavirvoitusjuomien valmistukseen.
Toisin sanoen, akun latautuessa akku sitoo ympäröivästä ilmasta hiilidioksidia ja purkautuessaan vapauttaa puhdasta hiilidioksidia teollisuuskäyttöön tai varastoitavaksi. Näin ilman puhdistamiseen tarvitaan vain sähköä. Verrattuna muihin hiilidioksidia sitoviin menetelmiin, akku on itseasiassa melko energiatehokas 278 kWh / sidottu hiilidioksiditonni.
http://news.mit.edu/2019/mit-engineers-develop-new-way-remove-carbon-dioxide-air-1025
Metalliton akku
Vedyllä toimivien kuorma-autojen valmistaja Nikola Motor on kertonut kehittävänsä akkua, joka tulisi mullistamaan sähköautoalan. Uusi akku omaisi kaksinkertaisen energiatiheyden, olisi 60% kevyempi ja puolet halvempi kuin nykyinen Teslan käyttämä litiumioniakku. Akkupaketti käyttäisi täysin uusia kennoja, jotka eivät käytä nikkeliä, kobolttia tai myrkyllisiä metalleja, joita tyypillisesti löytyy litiumioni-kennoista.
Uuden teknologian akku nostaisi sähköautojen toimintamatkaa nykyisestä noin 480 kilometristä aina 960 kilometriin per latauskerta. Akun koko ja paino kasvaisivat vain vähän, jos ollenkaan. Koska materiaalia ei tarvitse louhia, akku on edullisempi. Uusi kenno olisi tarkalleen 50% halvempi / kWh:a kohden kuin litiumioniakku. Lisäksi kaivostoiminnan ympäristöhaitat vähenevät.
Nikola ei ole paljastanut yksityiskohtia, mutta prototyypin pitäisi tulla 2020 vuoden lopulla. Valmistajan mukaan muut tekivät virheen yrittäessään löytää ratkaisun olemassa olevista teknologioista, kun puhtaalta pöydältä suunnittelu mahdollistaa voittavan akkuteknologian.
https://www.forbes.com/sites/alanohnsman/2019/11/19/hydrogen-truckmaker-nikola-claims-it-has-breakthrough-battery-techand-doesnt-care-if-youre-skeptical/#27e5669c157a
Tulevaisuuden akkumateriaalit merivedestä
Yhdysvaltalainen IBM Research-instituutti on kehittämässä akkua, joka metallittoman akun tavoin poistaisi tarpeen raskaiden metallien, kuten koboltin ja nikkelin käytölle. Uudella akkukemialla IBM pyrkii eroon raskasmetallien kaivostoiminnan saatavuus- ja eettisistä ongelmista (lapsityö, korruptio, saastuttavuus). Mielenkiintoisin seikka akussa on käytettävien materiaalien erottelu merivedestä.
Ensimmäisten testien perusteella todettiin, että kyettäisiin ohittamaan litiumioniakku alhaisemmalla hinnalla, nopeammalla latausajalla, korkeammalla teho- ja energiatiheydellä sekä elektrolyytin alhaisella syttyvyydellä.
Akun katodi ei käytä siis nikkeliä eikä kadmiumia ja sen elektrolyytti on turvallista nestettä, jolla on korkea leimahduspiste. Innovaatio lupaa suurta potentiaalia erityisesti sähköautoalalle, jossa akun syttyvyys, hinta sekä latausaika näyttelevät suurta roolia. IBM:n akun luvataan saavuttavan 80% varaustaso ainoastaan viiden minuutin latausajalla. Lisäksi akku kyetään suunnittelemaan pitkäikäiseksi, joka tekee siitä hyvän vaihtoehdon esimerkiksi uusille energiainfrastruktuureille, joissa säilyvyys ja stabiilius ovat pääroolissa.
https://www.ibm.com/blogs/research/2019/12/heavy-metal-free-battery/

Trendit kehittävät akkuja
Ympäristöystävällisyys akkujen valmistamisessa, käytössä sekä uusiokäytössä on yksi suurimpia akkuteollisuuteen vaikuttavia trendejä. Taistelussa elinympäristömme säilymisen puolesta tulevaisuuden akkujen tulee olla raskasmetallivapaita, mutta tarjoten kuitenkin ennennäkemätöntä potentiaalia käyttäjälleen. Akun raaka-aineiden tulee olla uusiutuvia, kestävällä tavalla hankittuja mutta akun tulee olla silti halvempi kuin koskaan ennen, jotta se kykenee syrjäyttämään vanhat, ympäristölle haitalliset akkumallit. Tehtävä ei ole helppo.
Akun valmistaminen ympäristöystävällisesti ja kestävällä tavalla on mahdollista, niin kuin kävi ilmi aiemmin mainituista innovaatioista. Akun massatuotanto ja sen vienti markkinoille on kohta, jossa varsinaiset haasteet iskevät: akun tulee täyttää kaikki vaadittavat osa-alueet, jotta se voidaan laskea tuotantoon. Lisäksi uusien akkujen rakentamiseen vaadittavat tehtaat ja tuotantolinjat vaativat vielä massiiviset panostukset.
Millainen on tulevaisuuden vastine litiumioniakulle?
Todennäköistä on, että raskasmetallit akun kennoissa tulevat korvautumaan jollain uusiutuvalla materiaalilla. Akun elinkaareen ei enää kuulu raaka-aineiden louhimista huonoissa olosuhteissa lapsityövoimalla ja sen kierrättäminen ja uusiokäyttö on entistä helpompaa. Raskasmetallien korvaaminen muilla materiaaleilla mahdollistaa nyt rajoituksien, kuten toimintamatkan, rikkomisen esimerkiksi sähköautojen osalta. Akkujen tarjotessa pidemmän käyttöajan entistä halvemmalla ja varmemmin, vähentyy myös bensiini- ja dieselmoottoreilla toimivien autojen tarve.
Tulevaisuuden akun pitäisi siis tarjota meille kaikkea enemmän, ympäristöystävällisemmällä tavalla.
Akkuteollisuudessa tapahtuva muutos ei rajoitu pelkästään akkuihin ja niiden käyttäjiin, vaan sillä on koko maapalloa koskeva vaikutus. Sähköautojen yleistyessä saastuttavat polttomoottorit vähenevät, energian varastointi suuressa mittakaavassa vähentää saastuttavaa energiantuotantoa ja akkujen uusiokäyttö vähentää tarvetta louhia raaka-aineita alati kasvavan kysynnän tarpeisiin.

Tämän vuoksi ladattavien akkujen ja paristojen suosiminen jo tänään niin käyttäjien, kuin jälleenmyyjienkin toimesta, ajaa akkuteollisuutta vääjäämättömästi entistä nopeammin kohti seuraavaa, vihreää akkua. Korvaa sinäkin kertakäyttöparistot ladattavilla akuilla ja vähennät jätekuormaa.
Proakku – Jesse
Lähteet:
- https://batteryuniversity.com/
- http://theleadsouthaustralia.com.au/industries/technology/new-battery-technology-could-slash-the-cost-of-electric-vehicles/
- http://news.mit.edu/2019/mit-engineers-develop-new-way-remove-carbon-dioxide-air-1025
- https://www.forbes.com/sites/alanohnsman/2019/11/19/hydrogen-truckmaker-nikola-claims-it-has-breakthrough-battery-techand-doesnt-care-if-youre-skeptical/#27e5669c157a
- https://www.ibm.com/blogs/research/2019/12/heavy-metal-free-battery/